KAPA HiFi PCR Kits
A second-generation polymerase engineered for extreme fidelity, robustness, and uniform amplification of AT- and GC-rich templates. 100x fidelity improvement compared to Taq polymerase.
KAPA Library Preparation Kits containing the novel KAPA HiFi DNA Polymerase are recommended for NGS library construction and amplifcation. Product information can be found here>>

KAPA HiFi DNA Polymerase is a novel, single-enzyme system that exhibits industry-leading performance when compared with other high fidelity polymerases and polymerase blends. KAPA HiFi has been engineered to have an increased affinity for DNA without the need for accessory protein domains. The intrinsic high processivity of KAPA HiFi DNA Polymerase results in significant improvements to yield, sensitivity, speed, target length, and the ability to amplify difficult templates (e.g GC-rich) – standardize your high fidelity PCRs with a single enzyme.
The engineered KAPA HiFi DNA Polymerase plus optimized buffer system offers:
- Superior fidelity – 100x improvement compared to Taq polymerase.
- Robust performance across a wide range of difficult templates, including both AT- and GC-rich amplicons.
- Consolidation of PCR protocols and reaction conditions with single enzyme.
- Long range amplification of complex targets – up to 15 kb from genomic DNA.
- High speed – reduce reaction times by up to 75%.
KK2101 KAPA HiFi DNA Polymerase with dNTPs (100 U) 100 U, 1 U/µL. Supplied with 5X Fidelity Buffer, 5X GC Buffer (both with Mg2+ at a 1X conc. of 2 mM), high-quality dNTP Mix (10 mM) and extra MgCl2 (25 mM). KK2102 KAPA HiFi DNA Polymerase with dNTPs (250 U) 250 units, 1 U/µL. Supplied with 5X Fidelity Buffer, 5X GC Buffer (both with Mg2+ at a 1X conc. of 2 mM), high-quality dNTP Mix (10 mM) and extra MgCl2 (25 mM). KK2501 KAPA HiFi HotStart DNA Polymerase with dNTPs (100 U) 100 units, 1 U/µL. Supplied with 5X Fidelity Buffer, 5X GC Buffer (both with Mg2+ at a 1X conc. of 2 mM), high-quality dNTP Mix (10 mM) and extra MgCl2 (25 mM). KK2502 KAPA HiFi HotStart DNA Polymerase with dNTPs (250 U) 250 units, 1 U/µL. Supplied with 5X Fidelity Buffer, 5X GC Buffer (both with Mg2+ at a 1X conc. of 2 mM), high-quality dNTP Mix (10 mM) and extra MgCl2 (25 mM). KK2601 KAPA HiFi HotStart ReadyMix (100 rxn) 100 x 25 µl reactions. Convenient 2X master mix containing KAPA dNTPs, reaction buffer, and Mg2+ at a 1X final conc. of 2.5 mM. Just add template and primers. KK2602 KAPA HiFi HotStart ReadyMix (500 rxn) 500 x 25 µl reactions. Convenient 2X master mix containing KAPA dNTPs, reaction buffer, and Mg2+ at a 1X final conc. of 2.5 mM. Just add template and primers. Product Description
KAPA HiFi DNA Polymerase is a novel proofreading polymerase that was the engineered to have high processivity, exhibiting significant improvements in yield, sensitivity, speed, target length, and ability to amplify difficult templates (e.g GC-rich) when compared to wild-type polymerases. The HotStart formulation of the enzyme increases reaction efficiency and sensitivity by eliminating spurious amplification products resulting from non-specific priming events during reaction setup and initiation.
KAPA HiFi PCR Kits include two buffers for optimal performance with difficult templates. Both buffers contain magnesium chloride allowing for convenient setup and robustness.
Product Applications
KAPA HiFi DNA Polymerase is designed for high fidelity PCR where amplified product is cloned for use in downstream applications such as:
- Site-directed mutagenesis
- Sequencing
- Protein expression
- Next-generation DNA resequencing
Amplicons generated with KAPA HiFi DNA Polymerase are suitable for routine downstream applications, including restriction enzyme digestion, blunt-end cloning, and sequencing.
Extreme fidelity
The increased processivity, strong proofreading activity, and optimized buffer system of the engineered KAPA HiFi DNA Polymerase results in superior accuracy for high fidelity PCR applications.
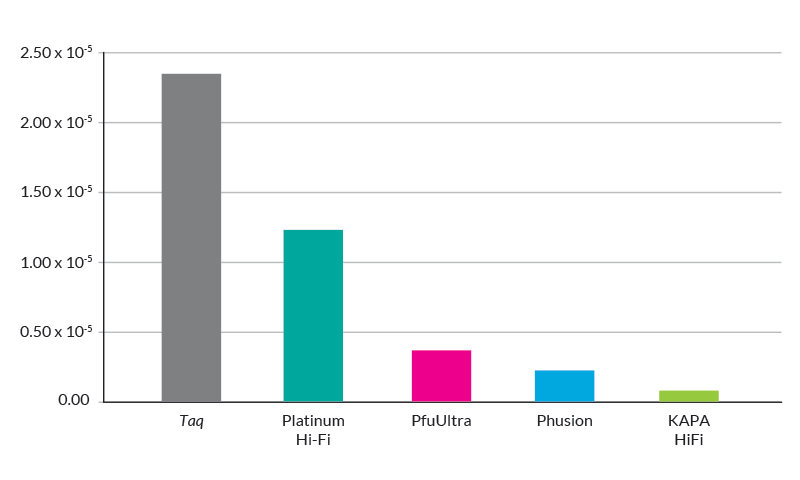
Figure 1. The error rate of KAPA HiFi is 100x lower than Taq polymerase, 40x lower than polymerase blends such as Platinum® Taq High Fidelity, 3x lower than Pfu Ultra, and 2x lower than Phusion.
Highest yield and sensitivity from long genomic target
Two-enzyme blend systems commonly used for long range applications are not suitable for high fidelity PCR because the error rates of polymerase blends are only 2 – 3x better than that of wild-type Taq (Figure 1).
The extreme processivity and robustness of KAPA HiFi DNA Polymerase offers the ability to perform high fidelity PCR on long and complex genomic templates with extreme sensitivity. The high speed of KAPA HiFi also allows significantly shorter reaction times for long range PCR.
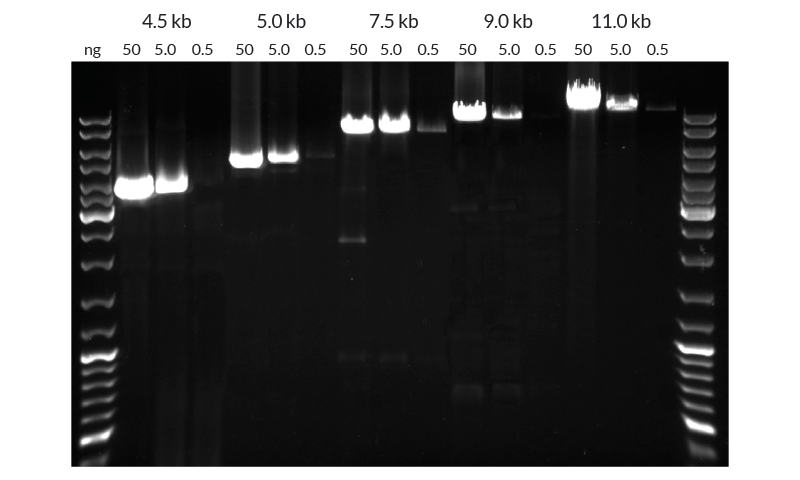
Figure 2. Amplification of hgDNA targets up to 11 kb. Each target was amplified from a descending range of template hgDNA concentrations (50 ng to 0.5 ng per reaction). Reactions (25 µl each) were performed using standard 3-step cycling profiles (35 cycles): 20 sec denaturation, 15 sec annealing, and 30 sec/kb extension time. Total reaction time for the 11 kb amplicon was 3h 50mins. 12.5 µl of each reaction was loaded on the gel.
Unrivalled success with difficult templates
KAPA HiFi DNA Polymerase exhibits robust performance on difficult templates. A panel of amplicons from human genomic DNA with a GC content ranging from 47% to 84% was used to compare the performance of KAPAHiFi™ HotStart and competitor kits. A polymerase with fusion technology in GC Buffer and a polymerase blend are only able to amplify targets up to 64% GC content. KAPA HiFi in GC Buffer achieves a 100% success rate with higher yields across all amplicons up to 84% GC content.
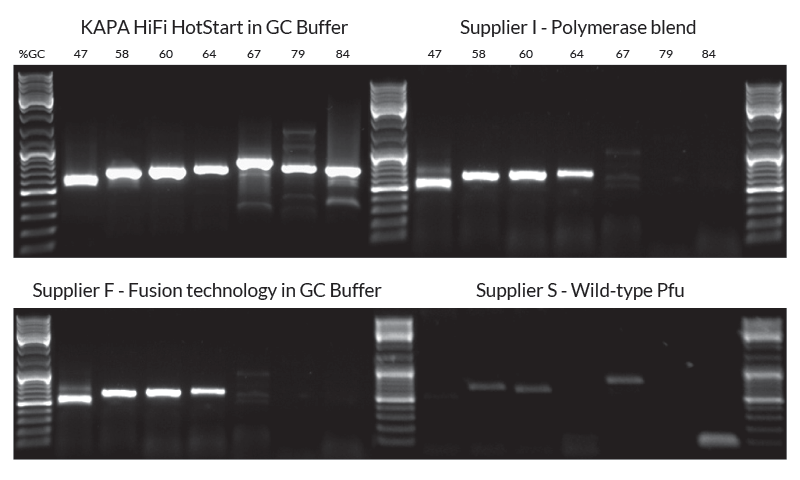
Figure 3. Amplification of AT- and GC-rich, single-copy human genomic targets. Seven single-copy gene fragments representing a range of GC content were used to compare the robustness of KAPAHiFi™ HotStart DNA Polymerase against a panel of competitor high fidelity DNA polymerases and polymerase blends. Amplicons range between 0.5 kb and 0.7 kb in length and ranged from 47% to 84% GC content. All reactions (25 µl each) contained 50 ng human genomic DNA as template and were performed using manufacturers’ protocols and buffers, with standard 3-step cycling profiles (35 cycles). 12.5 µl of each reaction was loaded on the gel.
Extreme sensitivity and fidelity
A major limitation for single-enzyme proofreading polymerases is poor sensitivity due to damaged nucleotides and primer degradation. The engineered KAPA HiFi DNA Polymerase exhibits dramatic improvements in sensitivity – outperforming fusion technology polymerases and polymerase blends.
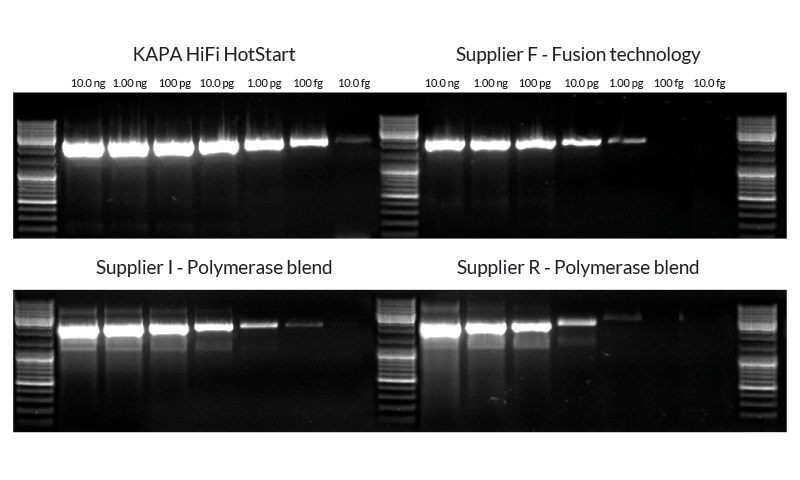
Figure 4. 2 kb lambda phage target amplified from a 10 fold dilution series starting from 10 ng down to 10 fg using KAPAHiFi™ HotStart DNA Polymerase with Fidelity Buffer, fusion technology polymerase, and polymerase blend systems. All reactions (25 µl each) were performed using manufacturers’ protocols and buffers, with standard 3-step cycling profiles (35 cycles). 12.5 µl of each reaction was loaded on the gel.